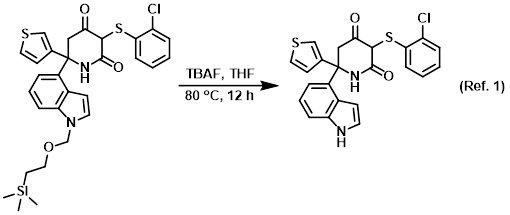
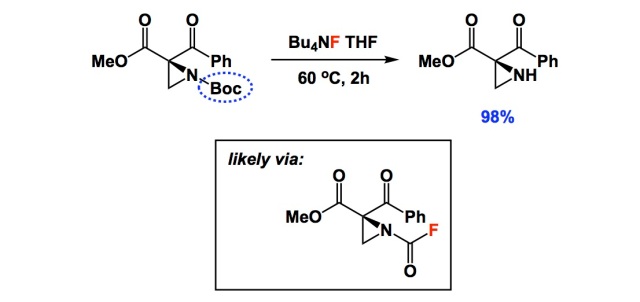
SciELO - Brasil - An Efficient and Chemoselective Deprotection of Aryl <i>tert</i>-Butyldimethylsilyl (TBDMS) Ethers by NaCN An Efficient and Chemoselective Deprotection of Aryl <i>tert</i>-Butyldimethylsilyl (TBDMS) Ethers by NaCN

Regioselective O2′,O3′‐Deacetylations of Peracetylated Ribonucleosides by Using Tetra‐n‐butylammonium Fluoride - Babu Kumar - 2014 - European Journal of Organic Chemistry - Wiley Online Library
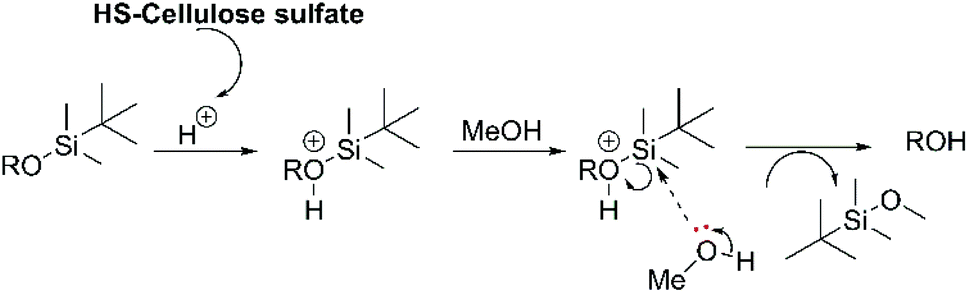
Highly sulphated cellulose: a versatile, reusable and selective desilylating agent for deprotection of alcoholic TBDMS ethers - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01438H
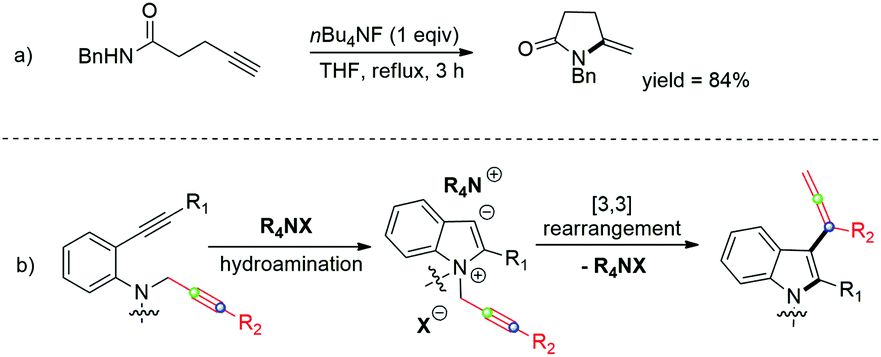
TBAF catalyzed one-pot synthesis of allenyl-indoles - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C7QO00414A

Tetrabutylammonium Fluoride as a Mild and Versatile Reagent for Cleaving Boroxazolidones to Their Corresponding Free α‐Amino Acids - Poulie - 2017 - European Journal of Organic Chemistry - Wiley Online Library