Novartis receives FDA approval for Beovu®, offering wet AMD patients vision gains and greater fluid reductions vs aflibercept – REGENHEALTHSOLUTIONS (RHS)

Aflibercept mechanism of action. Aflibercept binds to VEGF A-D and PIGF... | Download Scientific Diagram

Beovu 120 mg/ml solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)
Novartis receives FDA approval for Beovu®, offering wet AMD patients vision gains and greater fluid reductions vs aflibercept | Inderes: Osakeanalyysit, mallisalkku, osakevertailu & aamukatsaus

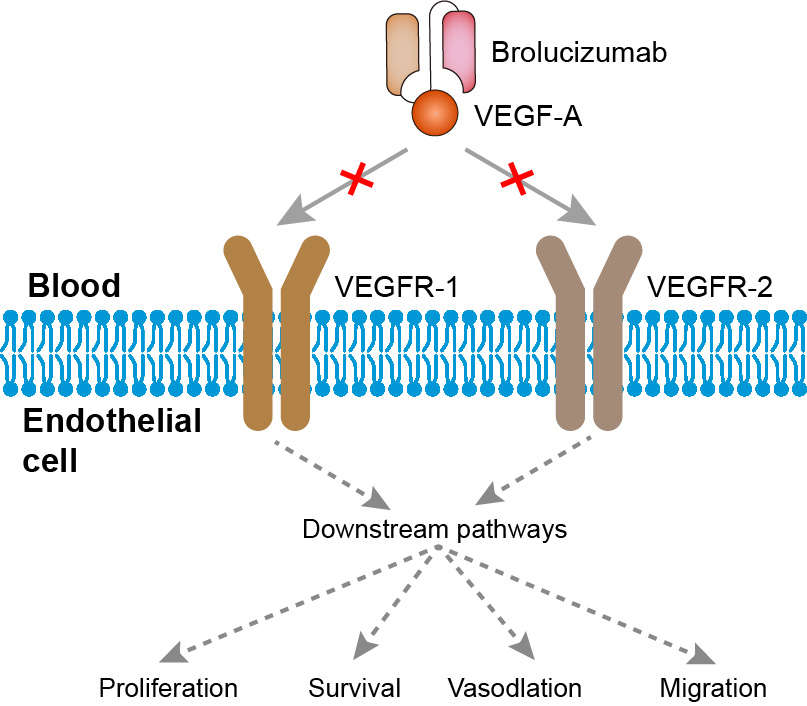
These highlights do not include all the information needed to use BEOVU safely and effectively. See full prescribing information for BEOVU. BEOVU® (brolucizumab-dbll) injection, for intravitreal useInitial U.S. Approval: 2019